Molecular Highlight: Unraveling the strokes of ion transporters in computers
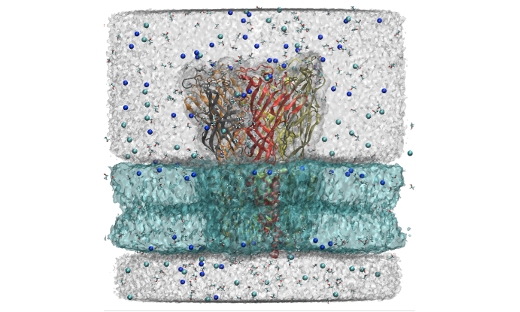
A ligand-gated ion channel embedded in a lipid bilayer. As neurotransmitters bind to the upper part of the receptor it will undergo a conformational change to open a channel through the membrane. This process is modulated by compounds such as alcohol or anesthetics that also bind in the transmembrane region. This is the way most drugs affect our central nervous system, and an area of great research and drug design interest.
As highlighted by the 2013 Nobel Prize for chemistry, life science and biomolecular modeling are some of the most important applications for molecular simulation. By simulating the motions of membrane protein transporters in close connection with experiments, we have been able to explain fundamental mechanisms of nerve signal propagation both inside and between cells. Simulations are even being used to validate experimental results to determine new structures, which has resulted in a number of high-impact results.
SeRC researchers in the molecular simulation community have been able to use molecular simulations to track the first-ever complete cycle of intermediate states as voltate-gated ion channels open in response to a nerve signal (1), something that has important implications for future drug design. Similarly, we have been the first to show that there are multiple separate “allosteric” binding sites that either amplify (potentiate) or decrease (inhibit) the response (2,3), which is the reason why we are aroused by some drugs but fall asleep from others. In addition, we have ongoing work that has shown that simulations can be used to predict the effects of mutations. Some of the greatest current challenges are to find ways to use this knowledge in order to design better anesthetics or even drugs that can be used to treat addiction disorders such as alcoholism.
SeRC has been a critical factor in this success, particularly since it has enabled long-term close collaborations between computational and applied research, both of which have resulted in several high-impact publications and helped achieve internationally leading competence for membrane protein simulations in Stockholm.
References
Henrion, U., Renhorn, J., Börjesson, S.I., Nelson, E.M., Schwaiger, C.S., Bjelkmar, P., Wallner, B., Lindahl, E.†, Elinder, F†. (2012) “Tracking a complete voltage-sensor cycle with metal-ion bridges”, Proc. Natl. Acad. Sci. U S A 109:8552-7
Howard, R.J., Murail, S., Ondricek, K.E., Corringer, P.-J., Lindahl, E., Trudell, J.R., Harris, R.A. (2011) “Structural basis for alcohol modulation of a pentameric ligand-gated ion channel”, Proc. Natl. Acad. Sci. USA 108:12149-12154
Murail, S., Howard, R.J., Brömstrup, T., Bertaccini, E.J., Harris, R.A., Trudell, J.R., Lindahl, E. (2012) “Molecular mechanism for the dual alcohol modulation of Cys-loop receptors”, PLoS Comput. Biol 8: e1002710
Nyblom, M., H. Poulsen, P. Gourdon, L. Reinhard, M. Andersson, E. Lindahl, N. Fedosova, and P. Nissen. (2013) “Crystal structure of Na+, K(+)-ATPase in the Na(+)-bound state.” Science 342:123-127
Andersson, M., D. Mattle, O. Sitsel, T. Klymchuk, A. M. Nielsen, L. B. Moller, S. H. White, P. Nissen, and P. Gourdon. (2014) “Copper-transporting P-type ATPases use a unique ion-release pathway.” Nature Struct. Mol. Biol. 21:43-48




